Talk:Cellular respiration
| Cellular respiration has been listed as a level-4 vital article in Biology. If you can improve it, please do. This article has been rated as C-Class. |
| WikiProject Physiology | (Rated C-class, Mid-importance) | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||
| WikiProject Molecular and Cell Biology | (Rated C-class, High-importance) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||
| This article is or was the subject of a Wiki Education Foundation-supported course assignment. Further details are available on the course page. Assigned student editor(s): Cariningram. Assigned peer reviews: Cariningram, Kieu Dinh. |
Original author's note[edit]
How does oxygen enter the mitochondria? This page's content comes from what I've learned in high school Biology. Some of it may be incorrect. Also, I'm guessing that there's just a little more that could be added. By all means, do so (of course, that's what the 'pedia is all about). --bdesham but like who even cares.
In biochemistry I was taught that cellular respiration is a process that describes the metabolic reactions and processes that take place in a cell to obtain energy via transferring electrons to an inorganic electron acceptor, usually oxygen. The reactions described in the "anaerobic respiration" section are not respiratory at all, rather they are fermentation reactions. Unless things have changed in the last 10 years, glycolysis (EMP pathway) serves as the front end for both respiration (TCA cycle and electron transport system) and fermentation (transferring electrons to organic molecules resulting in things like ethanol and lactic acid). Can others comment on the basic definition of respiration? Tokidoki25(Daisy Martinez) User Review: I thought that this article was VERY helpful, but some of the diagrams were very confusing. Rate! Review Written:December 8,2011 @ 3:23 Nbjansen 04:18, 11 March 2007 (UTC) :)
Table / flow chart[edit]
I've added a basic diagram covering the subprocesses of aerobic respiration.I've done it as a table rather than uploading the whole thing as a .png so that others can easily modify the info. However looking at the edit page the table looks complicated and offputting. If anyone wants to amend the content of the diagram but is put off editing the table by all the ugly HTML by all means let me know on my talk page and I will edit the table for you. Theresa knott 13:56 6 Jun 2003 (UTC)
- Yeah, that table is kinda offputting. I've made a flow chart in PNG format; if anyone needs/wants to change it, visit User Talk:Bdesham and say so. --bdesham 19:43 9 Jun 2003
i agree...
What about Aerobic Respiration in Bacteria[edit]
Bacteria of course do not have mitochondria so this discussion to "cellular respiration" is being diverted from its original search on Aerobic Respiration. This is a big gap to those who want to know what microorganisms are doing to get their ATP. — Preceding unsigned comment added by 172.78.42.204 (talk) 01:15, 1 February 2018 (UTC)
==Incorrect number of ATP?== The net gain of ATP is both 38 ATP as well as 36ATp. It actually depends on the shunt which is active(malate aspartate shunt=38 atp & glycerol phosphate shunt=36 atp). I wasn't going to say anything, since my knowledge is limited to high school bio, but when I see this page was also made with that knowledge...we were taught a net gain of 36 ATP, not 38...perhaps you forgot to subtract the 2 used in glycolysis? EDIT: In fact, the glycolysis article agrees with me--kreb's and the ETC makes 34 ATP/glucose. 24.218.58.113 19:39, 26 Nov 2004 (UTC). Further edit: the previous was me, I am a new user. After referencing my biology textbook, the real answer (I believe) is that 38 ATP is the *optimal* gain, generally not realized due to such losses as the energy needed to move pyruvate into the mitochondria. I will make a minor edit to reflect this; please correct me if this is the wrong action. Endersdouble 19:47, 26 Nov 2004 (UTC)
- Hmm... looking back at my notes from HS Biology, they say that the total gain of ATP from glycolysis and aerobic respiration is 38 (2 from glycolysis and 36 from aerobic respiration). I googled for "cellular respiration", though, and I found [1]. If you look under the "How many ATPs?" section, it says that the theoretical total is 38, but that due to conditions the number rarely exceeds 30. I'll look into this more when I have time. Cheers! --bdesham 19:49, 26 Nov 2004 (UTC)
This is how it works: In vitro (in a test tube with every ezymers, substrates at the right conditions), a biochemist can make 38 molecues of ATP from a molecule of glucose. However, in a eucaryotic cell, Glycolysis (which produces ATP and NADH)occurs in the cytoplasm while respiration (and the recycling of NADH) occurs inside the mitochomdria. And this is the problem: -- All the NAD in the cytoplasm would become NADH and glycolysis will stop due the the lack of NAD; Therefore, NADH in the cytoplasm must be transported into the mitochondria to unload its protons and electons (i.e NADH--> NAD). This transporting cost 2 ATP; thereforem in eucaryotes, we said they produce 36 ATP. However, these type of calculation is meaningless-- If glucose are committed to produce ATP and CO2, all living organisms will be the same- pails of ATP! The fact is, a cell will not produce a single molecue of ATP more than it need.
- Ok so looking at this it all fine and dandy but im taking bio right now and im preety sure it is 36 ATP but then aging maby im wrong this seems to be quite a perdiciment. —Preceding unsigned comment added by 67.58.207.41 (talk)
- 38 and 36 are purely theoretical numbers; read the section Theoretical_yields. But if we have to go with 36 or 38 then 36 is a more accurate estimate (but still pretty bad) of the most ATP that can be produced from a molecule of glucose. David D. (Talk) 15:36, 3 October 2006 (UTC)
- Even if 36 is more accurate than 38, the fact remains that the numbers are purely theoretical, so how close they are to the actual number of ATP produced should not be relevant. From what I have studied in Biology, 38 is the maximum number of ATP molecules that could potentially be produced, hence the theoretical number of ATP molecules produced by one molecule of glucose should be 38. EatMana 17:14, 24 January 2007 (UTC)
- This is not correct. 38 is impossible since the two NADH are outside the mitochondria. When the text books use 38 they assume those NADH can be used inside the mitochondria. That is wrong. If that is what your text book says then write to the author and get him/her to correct the error. David D. (Talk) 05:46, 25 January 2007 (UTC)
- Even if 36 is more accurate than 38, the fact remains that the numbers are purely theoretical, so how close they are to the actual number of ATP produced should not be relevant. From what I have studied in Biology, 38 is the maximum number of ATP molecules that could potentially be produced, hence the theoretical number of ATP molecules produced by one molecule of glucose should be 38. EatMana 17:14, 24 January 2007 (UTC)
- 38 and 36 are purely theoretical numbers; read the section Theoretical_yields. But if we have to go with 36 or 38 then 36 is a more accurate estimate (but still pretty bad) of the most ATP that can be produced from a molecule of glucose. David D. (Talk) 15:36, 3 October 2006 (UTC)
Stryer has been reporting about 30 ATP for many years now based on a 1991 Biochemistry paper 30:3576, by Hinckle, Kumar, et al. Most low level textbooks and teachers just keep niggling over the 36 or 38 without realizing that it's really changed. I changed the numbers to correspond to Stryer's numbers on page 552 of his 3rd edition. Eperotao 08:57, 21 July 2007 (UTC)
Just changed the total from 42 to 36 ATP. Previously written was 8 NADH generated from TCA cycle, this is incorrect according Lippincotts. There's actually only 6 NADH generated. (3 for each pyruvate). As for 38 vs 36, it depends on whether you are using the malate shuttle or the glyceraldehyde shuttle. —The preceding unsigned comment was added by 162.129.60.86 (talk) 20:37, August 21, 2007 (UTC)
- Looks like you corrected recent vandalism, it was 6 and 36 a while back. David D. (Talk) 21:40, 21 August 2007 (UTC)
Actually if you want to be specific, the total number of ATP molecules generated from one molecule of Glucose-6-phosphate is 30, plus 2 molecules of GTP. This is different than what has previously been stated because older figures rounded the actual number of ATP per NADH and FADH(2H) to 3 and 2 respectively. However, NADH account for 2.5 molecules of ATP and FAD(2H) account for 1.5. This is because NADH is oxidized by complex I of the electron transport chain, resulting in 4 hydrogen protons being pumped across the membrane at that complex. FAD(2H), however, deposits its electrons into complex II, which pumps no protons across the inner mitochondrial membrane. Thus, FAD(2H) does not contribute any protons to the proton motive force before complex III. Since it takes 4 hydrogen protons to create 1 ATP (3 protons for the ATP synthase and 1 for the ATP-ADP translocase), FAD(2H) will produce 1 less ATP. The numbers should not be rounded up because of the relatively recently described activity of Complex IV, which only pumps 2 protons across the inner mitochondrial membrane, and uses 2 protons in the reduction of molecular oxygen. Thus, the action of Complex IV can only provide enough protons to synthesize .5 molecules of ATP. —Preceding unsigned comment added by 98.249.5.218 (talk) 00:48, 2 October 2009 (UTC)
Hi, just to throw my idea's in, the current course im doing has tought us (as recently as today) that the theoretical net ATP is 38 and the actual is 32, the only difference is that redNAD and redFAD produce only 2.5 and 1.5 per pyruvate, respectivley. What do you make of this.--79.77.65.236 (talk) 23:59, 4 February 2010 (UTC)
Should this be merged with "Glycolysis?"[edit]
Reading this article and Glycolysis, I've noticed that they duplicate a lot of information. Should they be merged?
- I think it would be better to de-emphasise glycolysis on this page. This page should be an overview whereas the glycolysis page should be more detailed. David D. (Talk) 20:06, 28 October 2005 (UTC)
- I agree, it's good to have separate ones. This summarizes and shows its place, glycolysis can be for more extensive detail and general interest not related to other cellular respiration processes. Tyciol 20:26, 5 October 2006 (UTC)
Link reaction[edit]
Shouldn't the link reaction be included here? although an apparently small step in the respiration and metabolic process it is none the less vital as without Acetyl CoA the Krebs Cycle could not occur. -Unknown
Efficiency of cellular respiration[edit]
The actual yield is closer to 30 ATP molecules. User:69.113.3.155
- By the way i do agree that there needs to be more work the inefficency but what is the basis for that number of 30ATP's per glucose being 'normal'? The inefficiency of the proton pumping, leakyness of the inner membrane to protons or that intermediates from glucose to CO2 are continually being syphoned off for other metabolic reactions? I'm not sure we want to put a number on this since it seems that the efficiency would vary depending on the other metabolic reactions occuring in a cell at any given time.
- Same with the efficiency of the ETC which may vary depending on the flux through the pathway. For example, I would imagine the efficiency would decrease during hypoxia or when ATP levels are high since the maximum PMF would be produced under those conditions. However, it may be highly efficient when a lot of oxygen and ADP are available.
- Do you have any more information? David D. (Talk) 22:50, 21 November 2005 (UTC)
- I agree; the energy charge (roughly the ratio of ATP to ADP and AMP) and availability of molecules or requirements of the cell in terms of biosynthesis, would affect how much of the glucose and its metabolites are actually shuttled into oxidative phosphorylation and ATP synthesis. Also, uncoupling proteins, e.g. UCP-1, have been found in the inner mitrochondrial membrane, and are believed to act as (tightly regulated) proton channels that allow protons to reenter the matrix, but without formation of ATP, like a sort of energy shunt. I think they are thought to play important roles in heat generation, metabolic energy processing, and are perhaps also involved in the development or control of conditions such as obesity (this article, for example, describes some of the UCP family of proteins and what their functions could be in living organisms). I also imagine that blocking the electron transport chain, removing electrons from the electron carriers, or lessening the proteins' abilities to pump protons may also reduce ATP synthesis further. Agaricus 17:34, 26 November 2006 (UTC)
Added a "See also" section[edit]
In the Electron transport chain discussion pages, we noted that there are lots of pages on this subject without adequate cross-referencing. I added a See Also section to help correct this problem. -Rozzychan 18:04, 23 June 2006 (UTC)
Revertion[edit]
- "Cellular respiration, involves the exchange of gasses (oxygen and carbon dioxide) between capillary beds in the systemic loop of circulatory system and the interstitial fluid of the tissues of the body."
I just deleted the sentence above since this article does not deal with this definition at all. Is there a cite for cellular respiration being a description of the extracellular gas exchange? Maybe this information is better suited to the disambiguation page? David D. (Talk) 22:52, 13 August 2006 (UTC)
Fat oxidation?[edit]
I'm hoping someone can help me understand where fatty oxidation occurs in this aerobic metabolism, as I can't find it mentioned in the article (are my observation skills bad?). Even if not mentioned, since fat metabolization does take part with oxygen, it might be relevant, and the role of glucos metabolism in running fatty acid oxidation processes. Tyciol 20:26, 5 October 2006 (UTC)
- Fatty acid oxidation is indeed aerobic in humans and animals, and begins with the transport of fatty acid chains into the mitrochondria by the "carnitine shuttle". The fatty acid chains are linked to Coenzyme A (CoA) inside the mitrochondrion to form an acyl CoA molecule, and a series of reactions, termed "beta-oxidation", then occurs. The beta carbon, carbon 3 of the fatty acid chain, is oxidised and hydrated to a keto group, and another molecule of CoA then cleaves this molecule between carbons 2 and 3. This forms a molecule of Acetyl CoA, which can enter the Krebs Cycle, and an Acyl CoA which is now 2 carbons shorter. The process is repeated until the fatty acid chain is completely degraded into Acetyl CoA, or for odd-chain fatty acids, Propionyl CoA. Propionyl CoA is converted in a series of reactions to Succinyl CoA, which is also an intermediate in the Krebs Cycle.
- Animals do not have the ability to convert Acetyl CoA to glucose (plants can do this by utilising the glyoxylate cycle, which animals do not possess the enzymes for), and so almost all the ATP energy obtained from fatty acid oxidation must come directly from the Krebs Cycle and oxidative phosphorylation, both of which require oxygen to function. This inability to synthesise glucose from Acetyl CoA means that glucose metabolism is essential for the oxidation of fatty acids. Oxaloacetate, the compound that Acetyl CoA condenses with to form Citrate in the first step of the cycle, must be present for the cycle to run. It can be generated from pyruvate by pyruvate carboxylase (generated from glucose, other carbohydrates, glycerol and some amino acids in glycolysis) as well as replenished by the addition of intermediates into the Krebs Cycle itself (such as from some other amino acids and odd-chain fatty acids). Without enough oxaloacetate, Acetyl CoA cannot then be metabolised by the Krebs Cycle, and instead is funneled into ketogenic pathways to form "ketone bodies", such as acetoacetate. During times of starvation, when glycogen and carbohydrate reserves are depleted, muscle tissue breakdown is very important because many amino acids can be converted to pyruvate or Krebs Cycle intermediates, and these can then be used to generate oxaloacetate to keep the Krebs Cycle running, as well as allowing gluconeogenesis to occur. (Most of this information I extracted from Biochemistry, 6th ed., Borg et al., Chapters 17, 22 and 27)) Agaricus 18:47, 26 November 2006 (UTC)
There are a few articles like this one, like Complete Glucose Breakdown, Respiration (physiology) and Carbohydrate catabolism that appear to cover similar areas vaguely, but in a rather disjointed and confusing fashion. I personally think these should be merged or their contents combined into one single respiration overview article (i.e. giving details on how all the different pathways are combined in a typical cell to generate energy). Links to all the major reaction pathways involved in respiration could then be placed on this single page, which I think would help to make it a little easier to get a general overview of the whole topic. Alternatively, these could be merged into the Cell metabolism topic, with that page being the overview, but I personally think that catabolism and anabolism are two different things, and should have at least one page covering each separately. Any opinions? Agaricus 18:47, 26 November 2006 (UTC)
Article Updating/Metabolic Integration[edit]
I've just edited the introductory paragraph to cover respiration in a more general way (rather than simply focusing on glucose and aerobic respiration), although I'm not sure if the style of writing is unclear or not (please let me know if it is).
As for the plan, I imagine the overall goal for this article would be to describe and link to each of the major metabolic pathways involved in cell respiration, but to do this in a general, integrated fashion. So, for example, we could first describe the outline pathways of glycolysis, the Krebs Cycle and oxidative phosphorylation as the "major" respiration pathways. Then, we could consider the entry points of other carbohydrates (including glycogen), amino acids and fat breakdown products into this outline scheme. The ATP generation chart in this article is useful, but perhaps it is more suited, in its full form, to being on the Oxidative phosphorylation page instead (since it is mostly specific to that particular process, rather than to respiration in general). In the end (although I doubt I'll actually do enough editing to play a major part in it), I imagine that we'd want all of the metabolism articles organised in this way, with a general overview page that covers all the major pathways at once, with links to the topics covering each of the pathways in more detail (I think it's harder to appreciate the complexity and interlinking of metabolic pathways without considering metabolism as a whole).
I'll start to make edits when I have the time, but if anyone else wants to do any editing, or has any suggestions to make about the "plan" I propose above, please feel free to do so. (Edit: Forgot the signature) Agaricus 20:07, 27 November 2006 (UTC)
Aerobic respiration[edit]
In Aerobic respiration 38 ATP molecles are made and in anaerobic respiration 2 are made. Aerobic respiration is for multicellular organisms and anaerobic respiration is for bacteria. 2 ATP molecules would not sustain our energy needs.
- There is no scenario where 38 ATP is possible. The two NADH from glycolysis can never be used to give 3ATP even if the whole system is running at maximum efficiency. Energy is needed to shuttle the NADH reducing energy to the ETC so electrons will not pass through complex one when glycolytic NADH is oxidised.
- Yeast are not bacteria and use anaerobic respiration. There are plenty of aerobic microbes too. Why do you assume that 2ATP from glycolytic oxidation could not be enough energy to sustain multicellular life? David D. (Talk) 06:07, 1 December 2006 (UTC)
Anaerobic respiration and fermentation are two different processes. Respiration is always a process where an electron transport chain is involved and an inorganic molecule (oxygen is most common but sulfate or nitrate works too, when the two later are used it is anaerobic respiration) is used as the final electron acceptor while fermentation lacks an electron transport chain and uses an organic electron acceptor. —Preceding unsigned comment added by 130.238.116.4 (talk) 08:30, 12 May 2009 (UTC)
Article merge[edit]
Instead of puting links to the real articles such as anerobic respiration, maybe someone should place a copy in this article.--75.28.161.31 03:59, 1 December 2006 (UTC)
- No, it would be too big. This article is an outline of the processes involved. David D. (Talk) 06:07, 1 December 2006 (UTC)
Merge with respiration (physiology) ?[edit]
Respiration (physiology) is a brief, over-arching wikipage that could accept cellular repiration quite nicely, since it overlaps it by talking about aerobic and anaerobic metabolism. Any ideas here? Rhetth 20:33, 21 December 2006 (UTC)
- I don't recommend it. Cellular respiration is a very large and specific subject, as any biochemistry student who has ever endured it knows very well. Really, the minute details of oxidative phosphoylation do not need to live in what should be a broader-scoped physiology article. – ClockworkSoul 23:44, 21 December 2006 (UTC)
Incorrect Information[edit]
I would like to clarify that under anaerobic respiration, you talk about a fermentation process. Note: anaerobic respiration and fermentation are two completely different processes. Anaerobic respiration is very similar to aerobic fermentation, utilizing the pyruvate decarboxylase step, entering the TCA cycle, and undergoing electron chain transport. The difference is that the final electron acceptor is a molecule other than O2. It can be a variety of molecules such as NO3-, SO42-, etc. Fermentation, on the other hand, undergoes glycolysis and then pyruvate can enter a variety of pathways, such as homolactic/heterolactic fermentation, mixed acid fermentation, and ethanol fermentation. And as for the number of ATP produced, many text books have a range from 36-38 ATP net production. And I don't agree that cellular respiration should be merged with glycolysis. Glycolysis is one of four steps in cellular respiration (4 if you count the conversion of pyruvate via pyruvate decarboxylase). Many a link can be established to glycolysis but I don't recommend merging the information. Ambrish100 03:14, 14 March 2007 (UTC)
Regardign your three points:
- I agree with the anaerobic respiration versus fermentation distinction.
- As for the 36-38, the text books are wrong. And some text books, such as Workd of the Cell, Becker et al. get it right.
- I'm not sure I understand the last point. Isn't this article is an overview of the four steps of cellular respiration, why wouldn't glycolysis be mentioned here? There is a seperate article on glycolysis that expands on the details.
David D. (Talk) 06:02, 14 March 2007 (UTC)
Response: For my last point, I was just agreeing with you in one of your previous responses. Ambrish100 19:20, 16 March 2007 (UTC)
It says where cellular respiration occurs in eukaryotic cells but it does not say where it occurs in prokaryotic cells.
Information Regarding Useful Energy[edit]
It would be nice if this article were to provide information regarding the percentage of energy unlocked through respiration which can then be used by an organism - percentages would be useful. —Preceding unsigned comment added by Save the truth (talk • contribs) 17:09, 3 September 2007 (UTC) ssssss —Preceding unsigned comment added by 69.243.43.82 (talk) 23:52, 16 October 2007 (UTC)
==Anareobic respiration==[edit]
Main Article needs to turn to {{main}}.68.148.164.166 (talk) 07:57, 14 January 2008 (UTC)
- Not sure I understand. What do you mean? -- Paleorthid (talk) 14:29, 14 January 2008 (UTC)
- Thanks David D., I see now. - Paleorthid (talk) 16:32, 14 January 2008 (UTC)
Anaerobic Respiration is not the same as Ferrmentation. This section of the article describes fermentation when the pyruvate that is produced after glycolysis is reduced by NADH to form lactic acid (lactic acid fermentation) or ethanol (alcoholic fermentation). The electrons are donated to pyruvate. Anaerobic respiration is similar to aerobic respiration. In anaerobic respiration, you would go through glycolysis, pyruvate oxidation, kreb cycle and then electron transfer chain just as you would in aerobic respiration with the difference that that the terminal electron acceptor is not oxgyen.
"Such organisms are typically found in unusual places such as underwater caves or near hydrothermal vents at the bottom of the ocean." +- 40% of bacteria present in a normal nitrifying biofilm are using an anaerobic pathway. Such organisms are everywhere and not only typical for unusual places. — Preceding unsigned comment added by 2A02:A03F:4A31:200:71AB:845D:28FA:F604 (talk) 16:51, 9 January 2020 (UTC)
Inorganic vs. Organic final electron acceptors[edit]
In the following statement, I had changed organic, to inorganic - but there might need to be some clarification. "There are organisms, however, that can respire using other inorganic molecules as electron acceptors instead of oxygen"
I think this might be the clarification: - For final electron acceptors in the electron transport chain in aerobic respiration, they are inorganic - For final electron acceptors in fermentation (which can be aerobic or anaerobic), they are organic (pyruvic acid or derivatives of pyruvic acid)
Salvoland (talk) 17:22, 7 July 2008 (UTC)
- I think it is talking about lithotrophs. Tim Vickers (talk) 17:37, 7 July 2008 (UTC)
Diagram[edit]
I put together this diagram for my biology class and felt that it might be of use. It may be somewhat inaccurate or unclear so please suggest any changes and I'll make them when I can. I've put it here but not added it to the article because I'm not sure where or how to fit it into the page. --RegisFrey (talk) 16:18, 16 July 2008 (UTC)
Looks great! A couple of comments:
- You have a typo in "fumarate"
- Would be better to shift the arrow so that water is reduced by complex IV, it looks at present as if this happens on the ATP synthase.
- I'd cut the term "transistional reaction" since this is not commonly used (I had to look it up!)
Thank you. Tim Vickers (talk) 16:34, 16 July 2008 (UTC)
Thanks for the quick response. I made some changes (and uploaded) based on your comments so I hope that helps. I notice the text is being weird in wikipedia's render ('acetyl-CoA' ignores it's path, overlaps, etc.). the simple SVG displays 99% correct in Safari 3 (only slight overlaps) and displays completely correctly in Firefox 3. Anyway that's what's changed. --RegisFrey (talk) 15:29, 17 July 2008 (UTC)
Merge with Plant respiration[edit]
Hi. I'm proposing that Plant respiration be merged into here. That stub seems to be about the cellular level of things and as far as I know (not being a botanist) cellular respiration in plants is the same as in animals or indeed in most eukaryotes. I would just do it, but I'm not positive that there's not a shade of difference, so I figured I'd see if there were any objections. —Elipongo (Talk contribs) 21:13, 24 September 2008 (UTC)
- Looking at the article, I'd just turn it into a redirect to this one. There's nothing that isn't here. Tim Vickers (talk) 23:11, 24 September 2008 (UTC)
what i think —Preceding unsigned comment added by 86.162.137.252 (talk) 17:32, 23 January 2009 (UTC)
Chemical equations[edit]
I have tried to balance your equation for glucose to pyruvate, with little success. You may wish to revise your content after viewing this page. While the general summarization of the process is correct, the end reaction only generates one proton and no water molecules. Pdorion (talk) 10:30, 3 February 2009 (UTC)
Adding a summary table[edit]
I have recently created a table in MS-Word summarizing anaerobic and aerobic breakdown of glucose. It includes columns such as step name, reactants, products, location, and ATP yield. I think it would be useful to place it right above "See Also". How do I submit it for review?
Rhopalodia (talk) 22:53, 5 May 2009 (UTC)
- Hi there, you'll need to format it in a form that you can add here first, see Help:Table for instructions. Tim Vickers (talk) 23:37, 5 May 2009 (UTC)
Looks messy but hopefully will work.
| Step | Step Name | Reactants | Products | Location | Notes | Yield |
|---|---|---|---|---|---|---|
| Anaerobic breakdown of glucose in yeast (without oxygen) | ||||||
| 1 | Glycolysis | C6H12O6 2 ATP 4 ADP 2 NAD 2 Pi | 2 C3H3OOH 2 ADP 4 ATP 2 NADH 2 H+ | Cytoplasm | 2 ATP used to phosphorylate glucose | 2 ATP |
| 2 | Fermentation | 2 C3H3OOH 2 NADH 2 H+ | 2 C2H5OH 2 CO2 2 NAD | Cytoplasm | ||
| Summary | C6H12O6 4 ADP | 2 C2H5OH 2 CO2 | Cytoplasm | 2 ATP | ||
| Aerobic breakdown of glucose (with oxygen) | ||||||
| 1 | Glycolysis | C6H12O6 2 ATP | 2 C3H3OOH 2 ADP | Cytoplasm | 2 ATP used to phosphorylate glucose | 2 ATP |
| 2 | Acetyl CoA formation | 2 C3H3OOH 2 CoA-S-H | 2 CoA-S-H-C2H3O 2 CO2 | mitochondria | Oxygen for CO2 comes from glucose | |
| 3 | Citric acid cycle | 2 CoA-S-H-C2H3O 6 H2O | 2 CoA-S-H 4 CO2 | mitochondria | Oxygen for CO2 comes from water | 2 ATP |
| 4 | Electron transport chain | 3 O2 10 NADH | 6 H2O 10 NAD | Oxygen for water comes from environment | ||
| 5 | Oxidative phosphorylation | 12 H+ 34 ADP | 34 ATP | 30 ATP from NADH (assuming 3 ATP/NADH) 4 ATP from 2 FADH2 (assuming 2 ATP/FADH2) | 34 ATP | |
| Summary | C6H12O6 3 O2 | 6 CO2 6 H2O | Cytoplasm and mitochondria | 38 ATP | ||
Evolution[edit]
I think we need a section about the evolutionary history of this process. Kernow (talk) 03:49, 16 August 2010 (UTC)
The synthesis of acetyl-CoA[edit]
I am a bit on confused when it comes to the creation of acetyl-CoA. As far as I understand, it is always created during cellular respiration as it requires oxygen to be formed from pyruvate. However, some lines also suggests that it may be created without.
This article (Cellular Respiration) states, in the section Citric acid cycle, that:
- "When oxygen is present, acetyl-CoA is produced from the pyruvate molecules created from glycolysis. Once acetyl-CoA is formed, two processes can occur, aerobic or anaerobic respiration."
This statement suggest that acetyl-Coa is always created, with or without the required amount of oxygen to support aerobic respiration. Thus, at the same time, it also suggests that both aerobic and anaerobic respiration makes use of acetyl-CoA.
The Wikipedia article about acetyl-CoA states that:
- "Acetyl-CoA is produced during the second step of aerobic cellular respiration, pyruvate decarboxylation, which occurs in the matrix of the mitochondria."
In the Cellular Respiration-article, in the section Aerobic respiration, it is also stated that:
- "... pyruvate enter the mitochondrion in order to be fully oxidized by the Krebs cycle."
Both these statements suggest that acetyl-CoA is created within the mitochondria. However, the acetyl-CoA-article-statement denies that acetyl-CoA is created in anaerobic respiration.
Further down in the Cellular respiration-article, it is stated in the section Fermentation that:
- "Without oxygen, pyruvate (pyruvic acid) is not metabolized by cellular respiration but undergoes a process of fermentation. The pyruvate is not transported into the mitochondrion, but remains in the cytoplasm, where it is converted to waste products that may be removed from the cell."
This statement does not mention anything about acetyl-CoA. It does, however, deny that the pyruvate (which is made into acetyl-CoA) enters the mitochondrion. While the absence of the mitochondrion is logical in anaerobic respiration, this statement opposes the first statement of acetyl-CoA being created in both aerobic and anaerobic respiration, as acetyl-CoA is created within the mitochondrion.
What I've written here is probably messy and hard to grasp, but could anyone please try to explain this and fix any errors? This is very confusing! Sky380 (talk) 18:40, 30 November 2010 (UTC)
New additional image - thoughts?[edit]
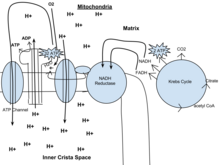
Not sure if anyone is watching, but, I'm the lead editor (teacher) of a WikiSchool project (WP:APBAPST), one of my students has attempted to create a simple overview of the aerobic processes of cellular respiration. The work is a bit rough, please feel free to remove the image if you find it to not be of the quality we expect on Wikipedia. Or leave comments for the author on her talk page. Thanks, Earthdirt (talk) 18:41, 25 May 2012 (UTC)
- First off, I think it's awesome that you're encouraging your students to contribute to Wikipedia. Nothing demonstrates understanding like teaching.
- I like the idea of the diagram, but I have a few minor suggestions.
- I'd fix the spelling of "mitochondria" at the top of the diagram. I'd also label ATP synthase, the inner mitochondrial membrane, the mitochondrial matrix, etc. And maybe clarify exactly how many of each molecule is consumed/produced for each turn of the cycle. But it's a great start!
- Thanks for the contribution,
- Rob Hurt (talk) 18:59, 25 May 2012 (UTC)
Table in Section 2: Efficiency of ATP production[edit]
Correct me if I'm wrong, but isn't oxidative phosphorylation the step after the Krebs cycle? If I'm right, shouldn't it be shown as a separate step? ZFT (talk) 22:40, 15 February 2015 (UTC)
Fatty acid discussion[edit]
Could we please have a section discussing the fatty acid metabolism aspect of aerobic respiration? This seems to focus primarily on glycolysis. More education regarding the different effect would be useful, particularly with many considering sugar/fat diet balance or wanting to know what would happen during fasting when glycogen stores drop and the body switches to fat stores for fuel. Or in those low-carb diets. If all we talk about is pyuvate entering mitochondria then we don't get to know when fatty acids enter them. 184.145.18.50 (talk) 15:48, 3 February 2016 (UTC)
Assessment comment[edit]
The comment(s) below were originally left at Talk:Cellular respiration/Comments, and are posted here for posterity. Following several discussions in past years, these subpages are now deprecated. The comments may be irrelevant or outdated; if so, please feel free to remove this section.
| high school/SAT biology content; changed importance to "high" for consistency with other cell metabolism subtopics - tameeria 14:59, 18 February 2007 (UTC) |
Last edited at 14:59, 18 February 2007 (UTC). Substituted at 11:08, 29 April 2016 (UTC)
In the Oxidative decarboxylation of pyruvate part, pyruvate dehydrogenase complex contents many copy of the three enzymes: Pyruvate Dehydrogenase (E1), Dihydrolipoyl Transacetylase (E2), and Dihydrolipoyl Dehydrogenase (E3). The prosthetic groups for E1, E2, and E3 are Thiamine pyrophosphate, Lipoamide, FAD respectively. More detail about pyruvate dehydrogenase complex could be found in this article [1]
In addition, Phosphofructokinase is the control enzyme which can stop the glucose catabolism when ATP, Citrate, and Pyruvate are built up. 09:08, 28 September 2016 (UTC)Kieu Dinh (talk)
References
- ^ Molecular structure of a 9-MDa icosahedral pyruvate dehydrogenase subcomplex containing the E2 and E3 enzymes using cryoelectron microscopy, https://www.ncbi.nlm.nih.gov/pubmed/16308322
External links modified[edit]
Hello fellow Wikipedians,
I have just modified one external link on Cellular respiration. Please take a moment to review my edit. If you have any questions, or need the bot to ignore the links, or the page altogether, please visit this simple FaQ for additional information. I made the following changes:
- Added archive https://web.archive.org/web/20080917123419/http://www2.ufp.pt/~pedros/bq/respi.htm to http://www2.ufp.pt/~pedros/bq/respi.htm
When you have finished reviewing my changes, you may follow the instructions on the template below to fix any issues with the URLs.
As of February 2018, "External links modified" talk page sections are no longer generated or monitored by InternetArchiveBot. No special action is required regarding these talk page notices, other than regular verification using the archive tool instructions below. Editors have permission to delete these "External links modified" talk page sections if they want to de-clutter talk pages, but see the RfC before doing mass systematic removals. This message is updated dynamically through the template {{sourcecheck}} (last update: 15 July 2018).
- If you have discovered URLs which were erroneously considered dead by the bot, you can report them with this tool.
- If you found an error with any archives or the URLs themselves, you can fix them with this tool.
Cheers.—InternetArchiveBot (Report bug) 19:37, 1 August 2017 (UTC)
External links modified[edit]
Hello fellow Wikipedians,
I have just modified one external link on Cellular respiration. Please take a moment to review my edit. If you have any questions, or need the bot to ignore the links, or the page altogether, please visit this simple FaQ for additional information. I made the following changes:
- Added archive https://web.archive.org/web/20070123180940/http://biology.clc.uc.edu/Courses/bio104/cellresp.htm to http://biology.clc.uc.edu/courses/bio104/cellresp.htm
When you have finished reviewing my changes, you may follow the instructions on the template below to fix any issues with the URLs.
As of February 2018, "External links modified" talk page sections are no longer generated or monitored by InternetArchiveBot. No special action is required regarding these talk page notices, other than regular verification using the archive tool instructions below. Editors have permission to delete these "External links modified" talk page sections if they want to de-clutter talk pages, but see the RfC before doing mass systematic removals. This message is updated dynamically through the template {{sourcecheck}} (last update: 15 July 2018).
- If you have discovered URLs which were erroneously considered dead by the bot, you can report them with this tool.
- If you found an error with any archives or the URLs themselves, you can fix them with this tool.
Cheers.—InternetArchiveBot (Report bug) 02:56, 6 December 2017 (UTC)
ATP synthase produces 1 ATP / 3 H+. No![edit]
This was based on (besides rather variable experimental results) the assumption that the synthase transports 9 protons and synthesizes 3 ATP per full cycle. The actual number of protons depends on the number of c-subunits in the Fo c-ring, which varies according to organism. Since 1999 it has been known that the yeast Fo c-ring has 10 subunits (Science 286, 1700–1705 (1999). https://www.ncbi.nlm.nih.gov/pubmed/10576729), and since 2010 that the vertebrate Fo c-ring has 8 (Bioenergetic Cost of Making an Adenosine Triphosphate Molecule in Animal Mitochondria. Watt, I.N., Montgomery, M.G., Runswick, M.J., Leslie, A.G.W., Walker, J.E. (2010) Proc.Natl.Acad.Sci.USA 107: 16823 PubMed: 20847295 https://www.ncbi.nlm.nih.gov/pubmed/20847295). Thus in vertebrate mitochondria the cost to make an ATP, including transport reactions, is 8/3+1 or 3.67 instead of 4. The 10 protons from oxidation of NADH yield 2.73 ATP instead of 2.5, and 6 protons from oxidation of succinate yields 1.64. These values are well within the experimental uncertainty expressed in the latest review of P/O ratios (P.Hinkle, Biochimica et Biophysica Acta 1706 (2005) 1 – 11 ) It would be good to redo this section specifying vertebrate mitochondria and using the "new" values. Eaberry (talk) 05:03, 19 March 2019 (UTC)
Or maybe just a note at the end indicating additional tweaks may be necessary- I can put that.Eaberry (talk) 16:36, 19 March 2019 (UTC)
a little suggestion of rewording[edit]
there's a little bit of repetition in the paragraph on the citric acid cycle in the aerobic respiration section at the moment so I'd suggest it be changed to something like this for some extra clarity sorry not everything here is going onto new lines as they should
Citric acid cycle Main article: Citric acid cycle This is also called the Krebs cycle or the tricarboxylic acid cycle. Acetyl-CoA is produced from the decarboxylation of the pyruvate molecules created in glycolysis. Once acetyl-CoA is formed, aerobic or anaerobic respiration can occur.[4] If oxygen is not present, fermentation of the pyruvate molecule will occur.
When oxygen is present, the mitochondria will undergo aerobic respiration which leads to the citric acid cycle. Here, acetyl-CoA is oxidized to CO2 while at the same time reducing NAD to NADH. NADH can be used by the electron transport chain to create further ATP as part of oxidative phosphorylation. To fully oxidize the equivalent of one glucose molecule, two acetyl-CoA must be metabolized by the Krebs cycle. Two waste products, H2O and CO2, are created during this cycle.
The citric acid cycle is an 8-step process involving 18 different enzymes and co-enzymes.[4] During the cycle, acetyl-CoA (2 carbons) + oxaloacetate (4 carbons) yields citrate (6 carbons), which is rearranged to a more reactive form called isocitrate (6 carbons). Isocitrate is modified to become α-ketoglutarate (5 carbons), succinyl-CoA, succinate, fumarate, malate, and, finally, oxaloacetate.
The net gain of high-energy compounds from one cycle is 3 NADH, 1 FADH2, and 1 GTP; the GTP may subsequently be used to produce ATP. Thus, the total yield from 1 glucose molecule (2 pyruvate molecules) is 6 NADH, 2 FADH2, and 2 ATP.
a clear summary similar to this would be good too: acetyl-CoA + oxaloacetate ---> citrate. citrate ---> isocitrate ---> α-ketoglutarate ---> succinyl-CoA ---> succinate ---> fumarate ---> malate ---> oxaloacetate — Preceding unsigned comment added by Lucas-O'D (talk • contribs) 19:51, 4 December 2019 (UTC)
Sjsj[edit]
Ydsgegehje ekeeu +(ùßçœlèç: Sachidanand gupta 2007 (talk) 13:39, 25 December 2019 (UTC)
- All Wikipedia vital articles in Biology
- Wikipedia level-4 vital articles in Biology
- Wikipedia C-Class vital articles in Biology
- Wikipedia C-Class level-4 vital articles
- C-Class Physiology articles
- Mid-importance Physiology articles
- Physiology articles about respiratory physiology
- WikiProject Physiology articles
- C-Class MCB articles
- High-importance MCB articles
- WikiProject Molecular and Cellular Biology articles

No comments:
Post a Comment